NO3 Lewis Structure How to Draw the Lewis Structure for NO3 YouTube
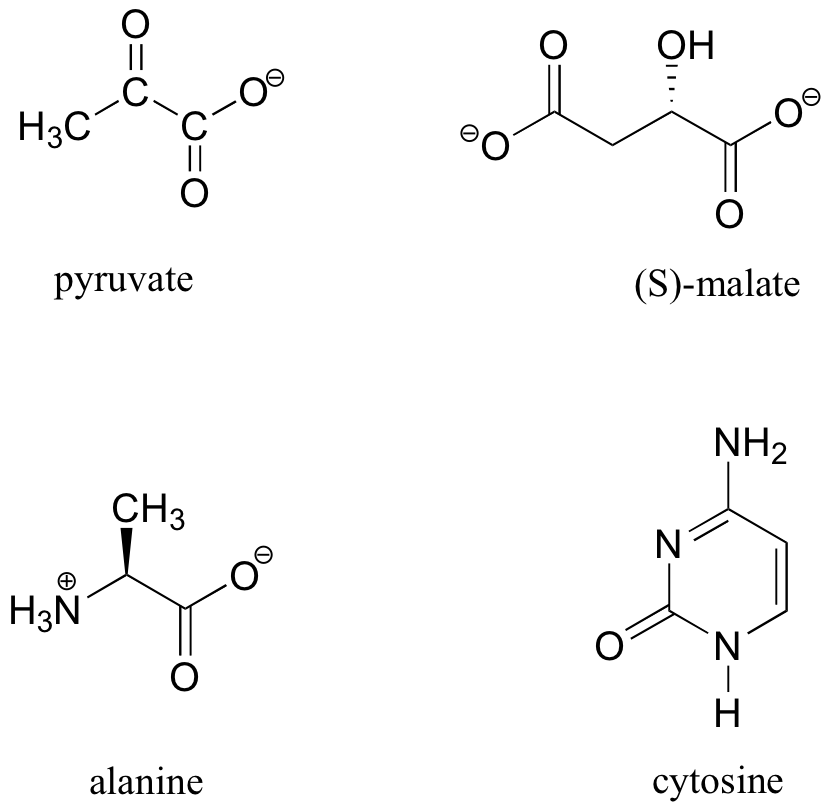
No3 Dot Structure
How to Draw the Lewis Dot Structure for NO3 - (Nitrate ion) - YouTube A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure.
Chem Molecular Shape (Molecular Geometry) Scientific Tutor
A simple procedure for writing Lewis Dot Structures is shown in this video.Several worked examples relevant to this procedure are given.http://chem-net.blogs.
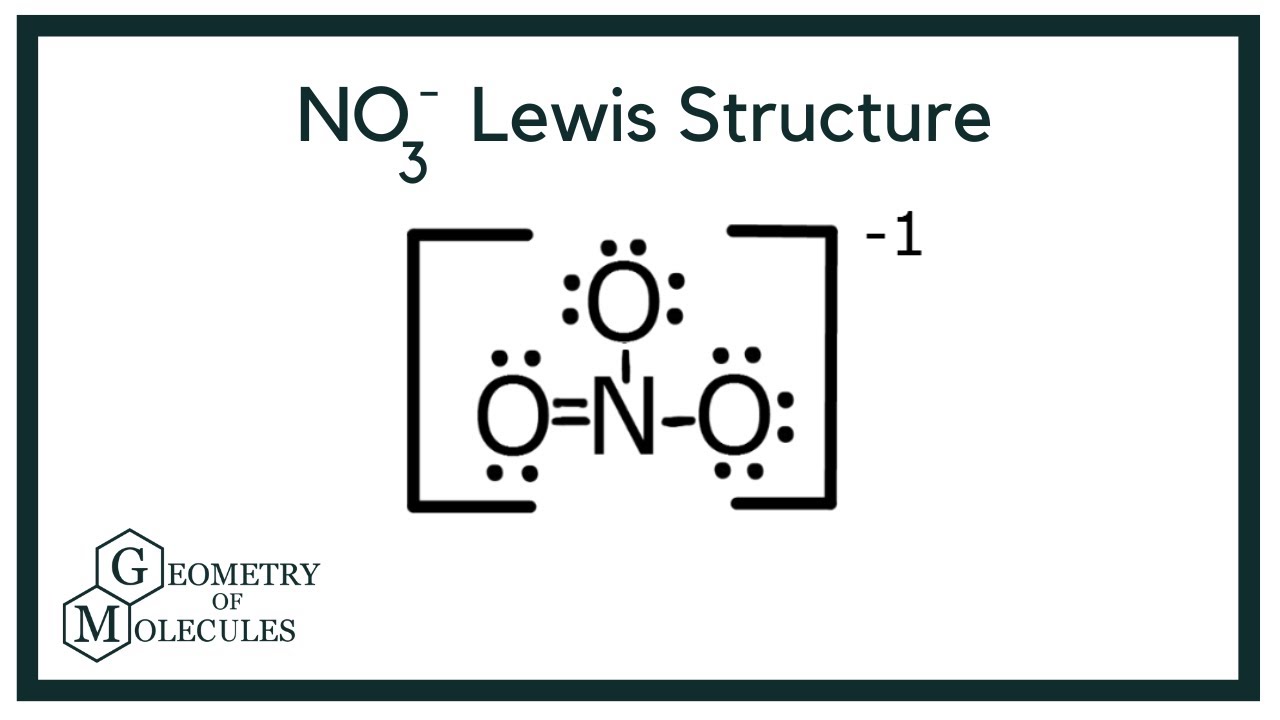
Top No3 Lewis Structure Molecular Geometry Bond Angles Background GM
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
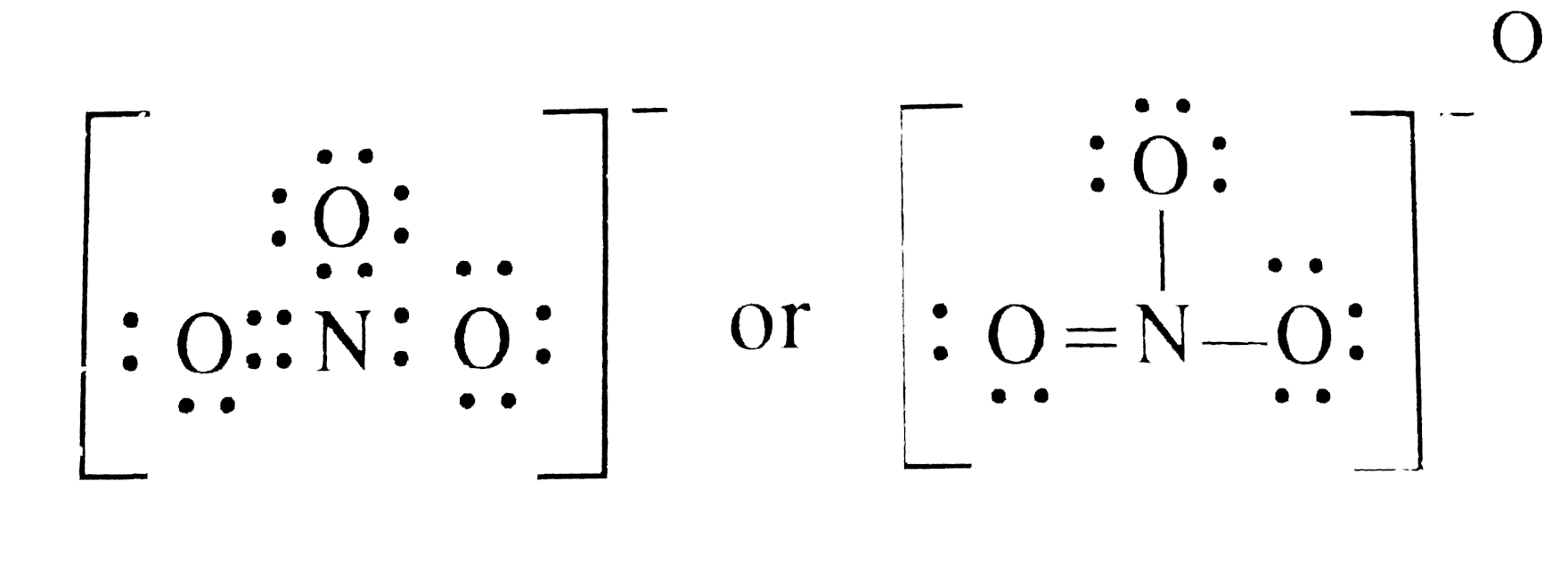
How To Draw The Lewis Dot Structure For No3 Nitrate Ion
How to draw lewis structure of NO3-? The Lewis structure of a nitrate [NO3]- ion consists of a nitrogen (N) atom and three oxygen (O) atoms. The nitrogen (N) atom is present at the center of the molecular ion, while three oxygen (O) atoms occupy terminal positions, one on each side.

How To Draw The Lewis Dot Structure For No3 Nitrate Ion
Lewis structure of NO3- ion (nitrate ion) contains one double bond and two single bonds between the Nitrogen (N) atom and Oxygen (O) atoms. The Nitrogen atom (N) is at the center and it is surrounded by 3 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.

Drawing Lewis Dot Structures For Covalent Compounds Youtube
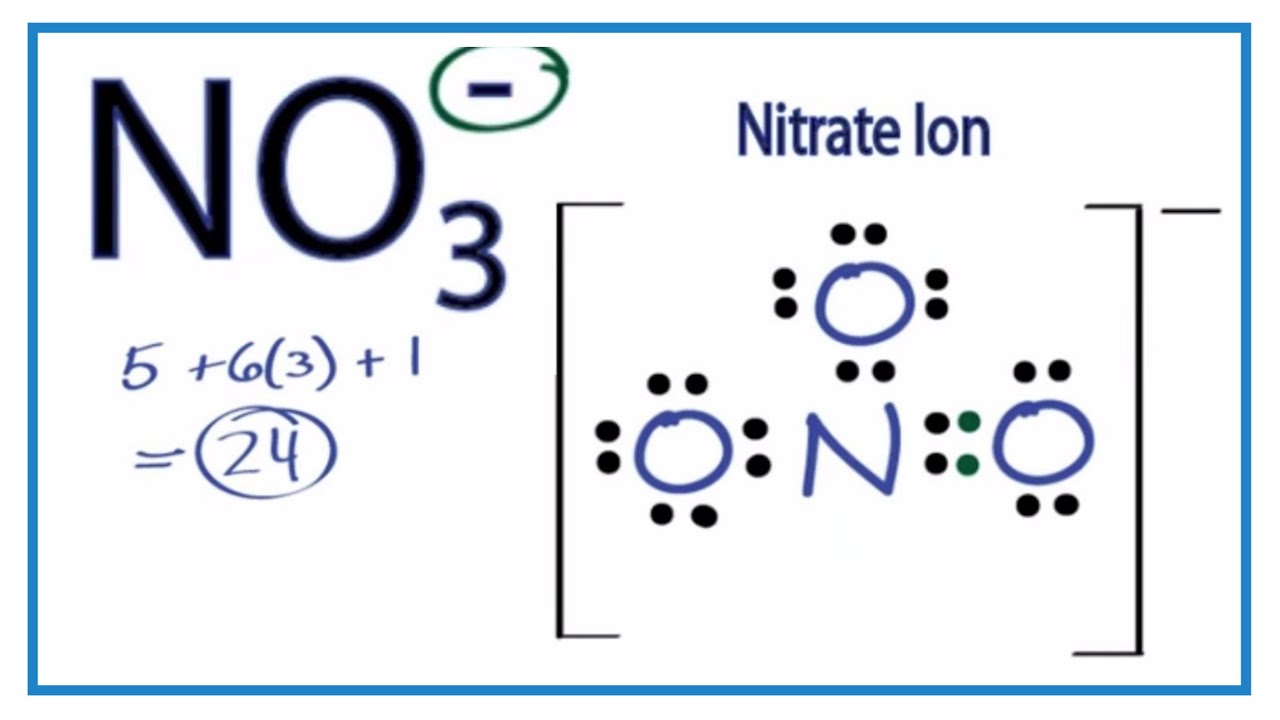
Drawing the Lewis Structure for NO 3- ( Nitrate Ion) Nitrates (salts with NO 3-) are frequently used in agriculture as a fertilizer. This is in part to their high solubility in water. There are 24 valence electrons available for the Lewis structure for NO 3-. Try to draw the NO 3- Lewis structure before watching the video.

NO3 Molecular Geometry / Shape and Bond Angles YouTube
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.
NO3 Lewis Structure Draw Lewis Dot Structure of Nitrate Ion YouTube
Lewis structure of NO 3- ion is drawn step by step in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the NO 3- lewis structure. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future.

NO3 Lewis Structure, Molecular Geometry, and Hybridization
Lewis Dot Structure of NO3- (Nitrate Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 294K views 12 years ago Every Video I quickly take you through how to draw the Lewis.

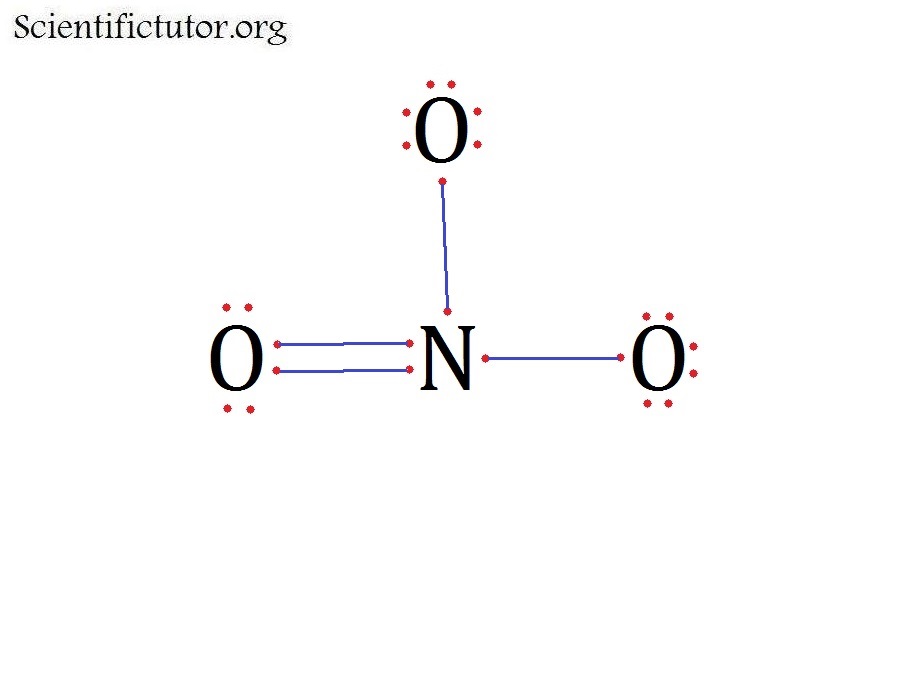
what is resonance?resonating structure of NO3 ion Brainly.in
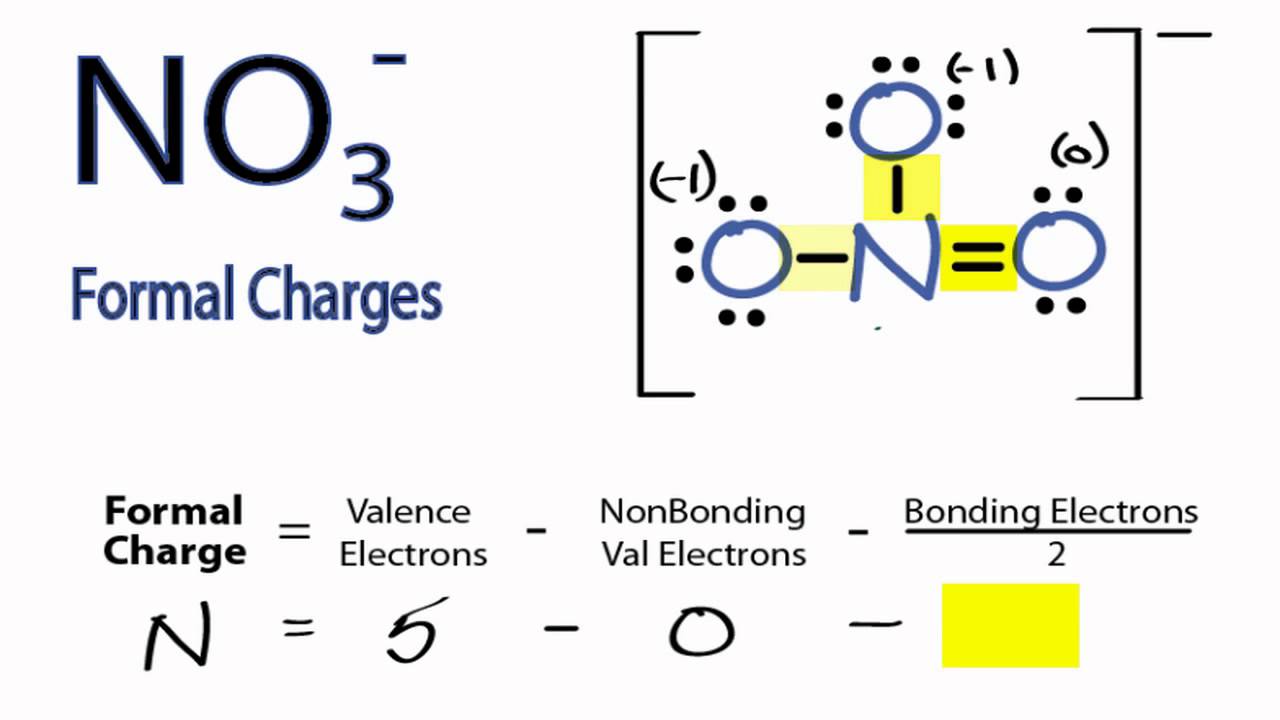
in the lewis structure for nitrate ion, NO3-, what is the total number of reasonable resonance structures that can be drawn? Three reasonable resonance structures can be drawn. Each of those resonance structures, two oxygen atoms have charges (each one has -1) and nitrogen atom has a +1 charge.
NO3 Lewis Structure How to Draw the Lewis Structure for NO3 YouTube
NO3- (nitrate ion) lewis structure has a Nitrogen atom (N) at the center which is surrounded by three Oxygen atoms (O). There is 1 double bond and 2 single bonds between the Nitrogen atom (N) and each Oxygen atom (O). There are 2 lone pairs on double bonded Oxygen atom (O) and 3 lone pairs on single bonded Oxygen atom (O).
no2 bond order
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.

Lewis structure of NO3 (Nitrate ion)Draw the Lewis dot structure of
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing.

Lewis dot structure of NO3 ion Nitrate ion lewis structure YouTube
How To Draw The Lewis Structure of NO3- (Nitrate Ion) The Organic Chemistry Tutor 7.27M subscribers Join Subscribe Subscribed 1.7K 148K views 3 years ago This chemistry video tutorial explains.

Lewis Structure NO3 plus dipoles, shape, angles, resonance and formal
Contents show Construction of NO3 Lewis Dot Structure 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell.

SOLVED Draw the Lewis Dot Structure for nitrate, NO31
GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are shared between two atoms as a covalent bond.