C2h2cl2 Lewis Structures
Lewis Dot Structure For C2h2cl2 Drawing Easy
Subscribed 1.2K views 1 year ago Lewis Structure CH2cl2 is a chemical formula for 1,2 dichloroethene. And to help you understand the Lewis Structure of this molecule, we are going to share.

C2h2cl2 Lewis Structure How To Draw The Lewis Structure
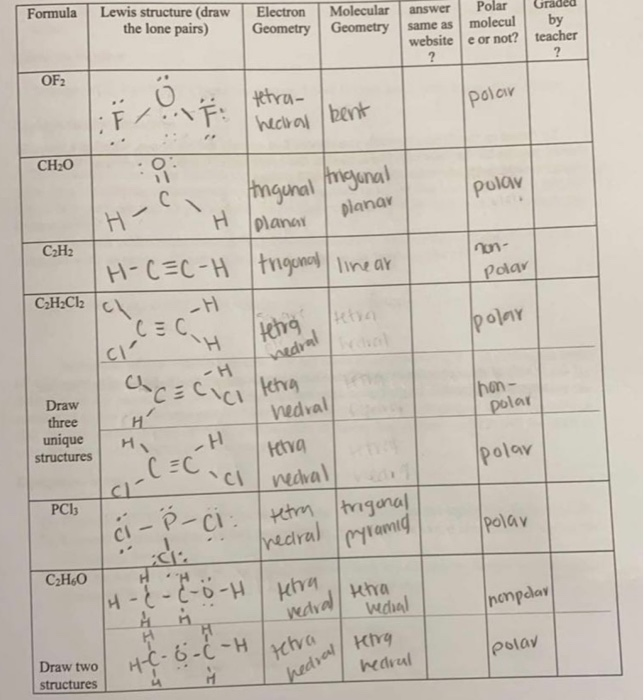
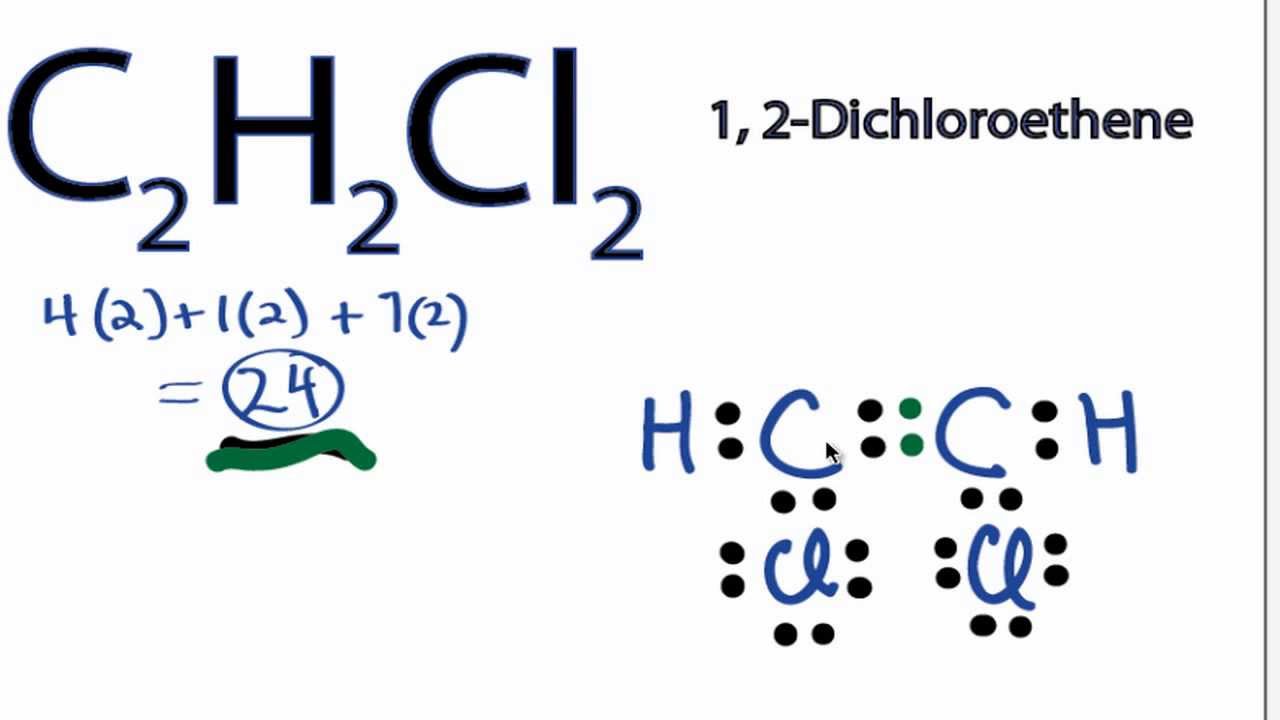
C 2 H 2 Cl 2 Lewis structure is made up of two carbon atoms, two hydrogens, and two chlorine atoms. Both carbon atoms are kept at the central position and other atoms are at the surrounding position in lewis's structure. The lewis structure of C 2 H 2 Cl 2 contains one double bond (C=C) and four single bonds.

C2H2Cl2Molecule Structure Royalty Free Stock Photography Image 9561677
With C 2 H 2 Cl 2 there are only single bonds. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Cl 2 Lewis structure. Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons to have a full outer shell.

Lewis Dot Structure For C2h2cl2 Bauman Atten1980
Furthermore, carbon bonds to carbon, giving acetylene a linear structure and a 180° bond angle. C2H2 Lewis Structure. The Lewis structure of C2H2 aids in the comprehension of the molecule's shape. The Lewis Structure of any molecule helps in understanding the atomic arrangement, valence electrons, and bond formation in the molecule.
Chapter 1112 Problems Solutions 1. cis and trans C2H2Cl2 has the
Contents show CH2Cl2 Lewis Structure The Lewis theory of chemical bonding—although quite primitive and the most limited theory on electronic structure—does help one to determine how valence electrons are arranged around the constituent atoms in a molecule. The purpose of this theory is to help visualize the chemical bonding of atoms in molecules.

Chemistry The Central Science 9780134414232 Exercise 43a Quizlet
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

C2h2cl2 Lewis Structure 3d
C2H2Cl2 (1, 2-dichloroethene) lewis structure has a double bond between the Carbon-Carbon atoms and a single bond between the Carbon-Hydrogen atoms and Carbon-Chlorine atoms. There are 3 lone pairs on Chlorine atoms (Cl).
C2h2cl2 Lewis Structures
In the C 2 H 2 Cl 2 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom and one chlorine atom, and on each chlorine atom, there are three lone pairs. C2H2Cl2 Lewis Structure: How to Draw the Lewis Structure for C2H2Cl2. Watch on Contents Steps
C2H2Cl2 Lewis Structure How to Draw the Lewis Structure for C2H2Cl2
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
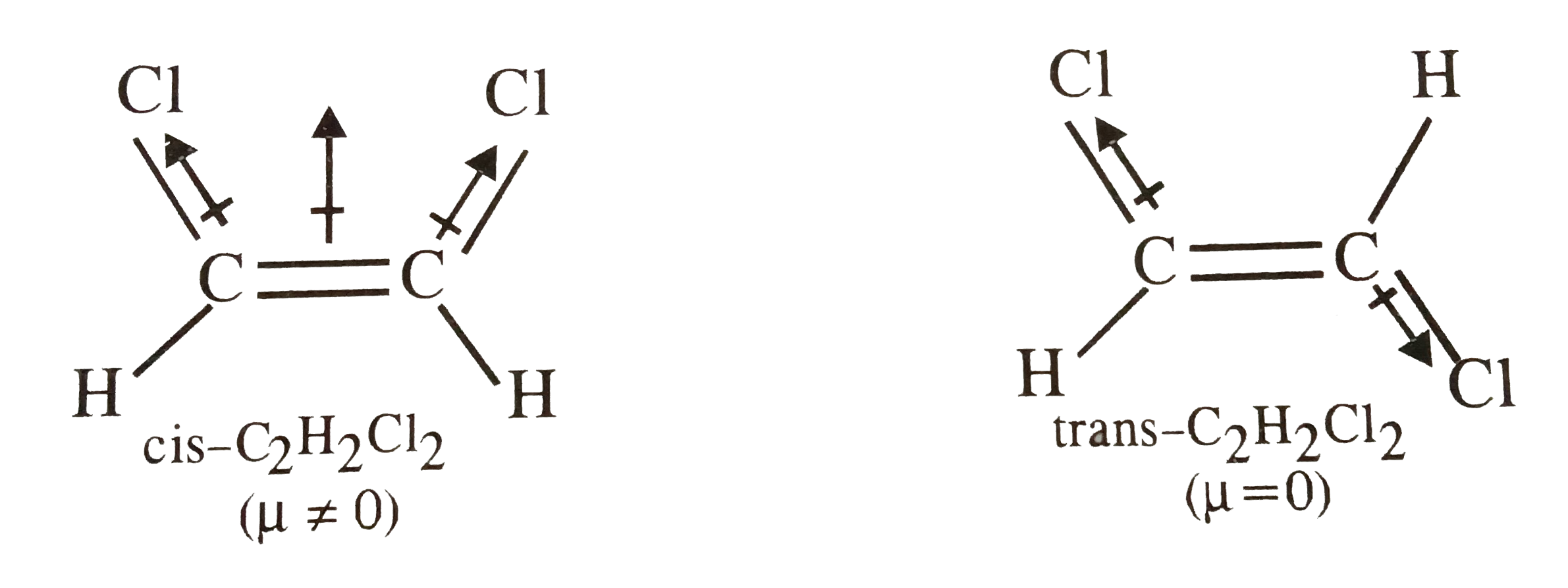
Lewis Dot Structure For C2h2cl2 Drawing Easy
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula C 2 H 2 Cl 2. They are both colorless liquids with a sweet odor.

Lewis Dot Structure For C2h2cl2 Drawing Easy
1,1-Dichloroethene. Molecular Formula CHCl. Average mass 96.943 Da. Monoisotopic mass 95.953354 Da. ChemSpider ID 13835316.

This is the correct Lewis structure for the molecule C2H2Cl2? True or
Lewis structure of C2H2Cl2 (1, 2-dichloroethene) contains a double bond between the two Carbon (C) atoms and a single bonds between Carbon-Hydrogen atoms and Carbon-Chlorine atoms. The Chlorine atom has 3 lone pairs. Let's draw and understand this lewis dot structure step by step.

6. Compound C2H2Cl2 Lewis Structure A Find all 3 Isomers Lewis
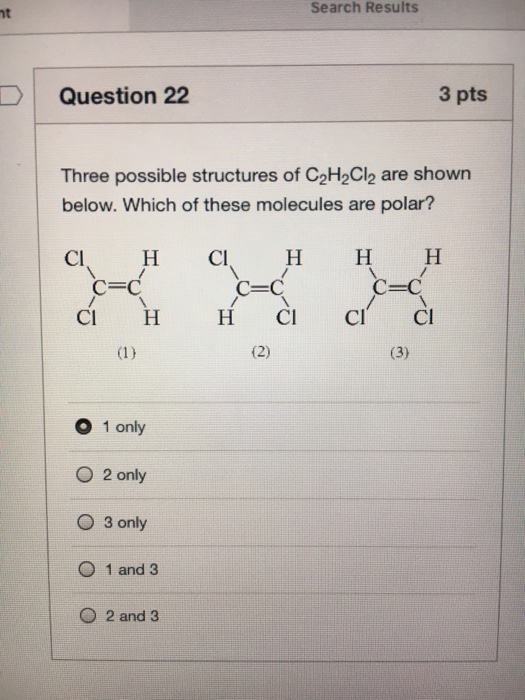
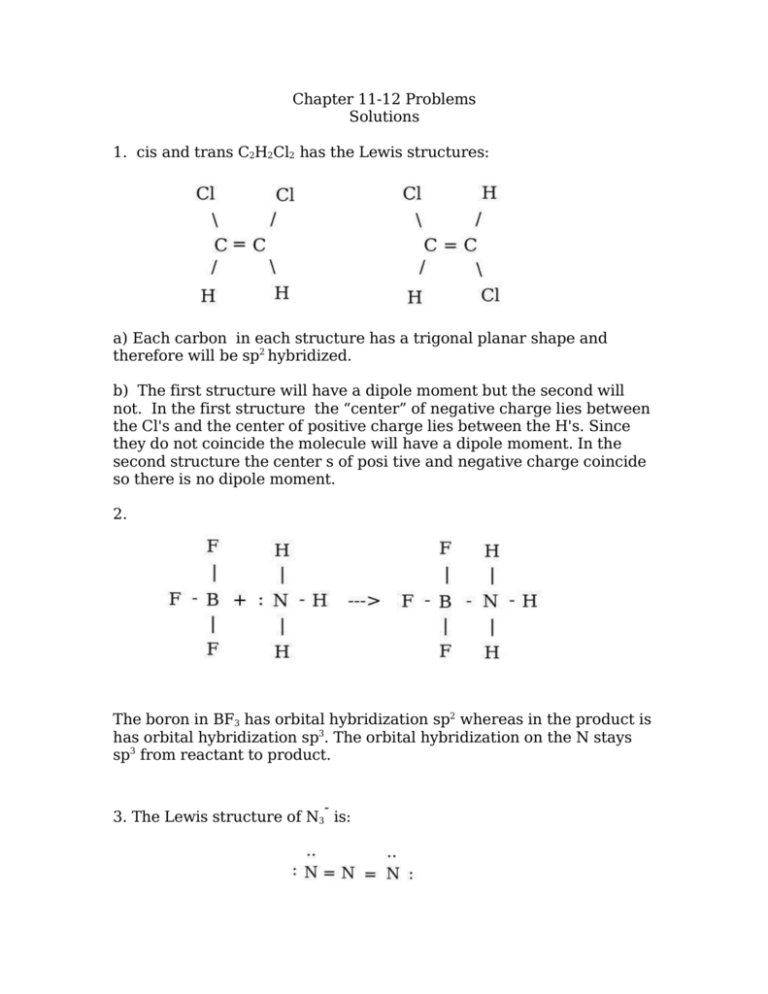
molecular geometries . A. b. trans-1,2-dichloroethene has no dipole moment and is nonpolar, while cis-1,2-dichloroethene has a dipole moment and is polar. Q9. Iodine reacts with fluorine to yield several compounds with the molecular formula \ (IF_n\) with \ (n\) varying from 1, 3, 5, and 7. Draw the Lew structure of each.

Solved Explain why one of the three structures for C2H2Cl2
A step-by-step explanation of how to draw the C2H2Cl2 Lewis Dot Structure (1,2-Dichloroethene). For the C2H2Cl2 structure use the periodic table to find the total number of valence.

C2H2Cl2 Lewis Structure How to Draw the Lewis Structure for C2H2Cl2
Lewis structure of C 2 H 2 Cl 2. The Lewis structure of C2H2Cl2 contains one double bond and four single bonds, with two carbons in the center, and each carbon is attached with one hydrogen and one chlorine. There are three lone pairs on each chlorine atom, and carbon atom and hydrogen atom do not have any lone pair.
a 2H2Cl2 Molecule 4 C2H2Cl2 Draw the Lewis Structure
A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the total.